Sensorised insoles for therapeutic monitoring of patients
STEP (Sensorised insoles for therapeutic monitoring of patients) project is designed to propose an affordable system for patient monitoring in a real-life environment. Based on sensorised custom-made insoles and integrated data analysis software, the system will support doctors providing a longitudinal analysis on their patients. This will allow an optimal decision making process ensuring the best outcome for patients.
| Partner | Role | Expertise |
|---|---|---|
| MADE | DIH | MADE provides a wide range of knowledge, methodologies, and digital tools that encompass the entire product lifecycle: from the design to the engineering, from the production management to the delivery to the customer, and the end of the product lifecycle. |
| medere | Technology provider | Medere is an Italian innovative start-up founded enthusiastically by three biomedical engineers. Their goal is to provide solutions and innovation for medicine and health. |
Business case
Several pathologies are not well monitored throughout their course. Doctors need a valid and reliable system to monitor patients remotely and to access longitudinal data with ease.
Solution
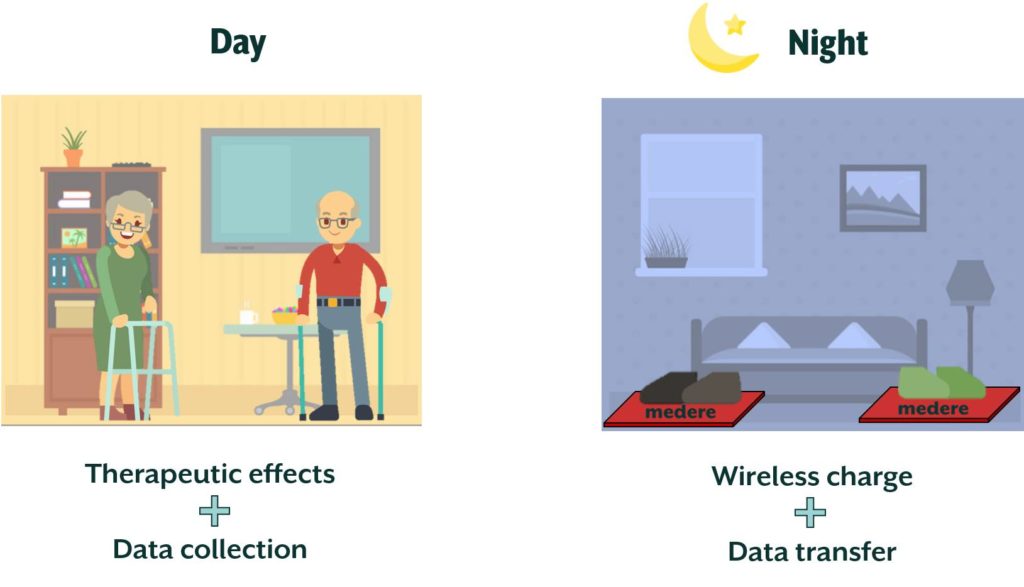
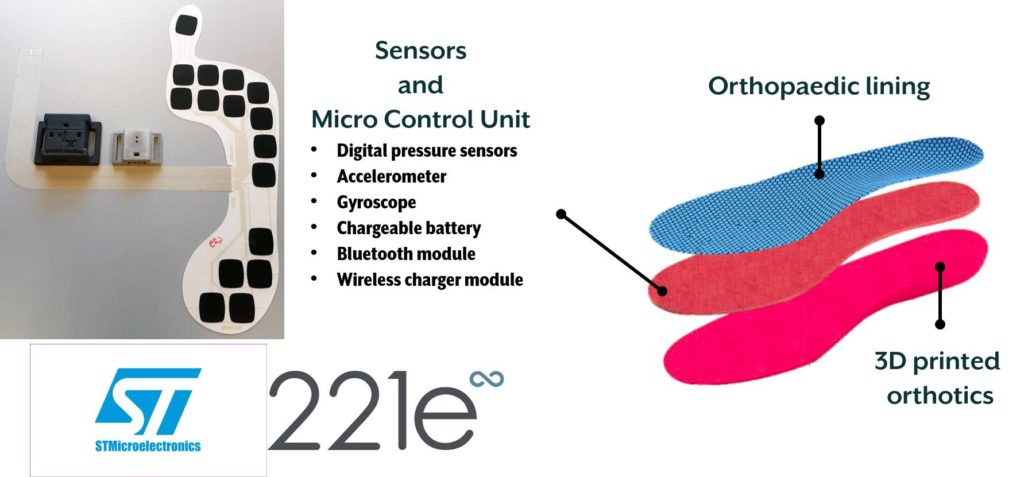
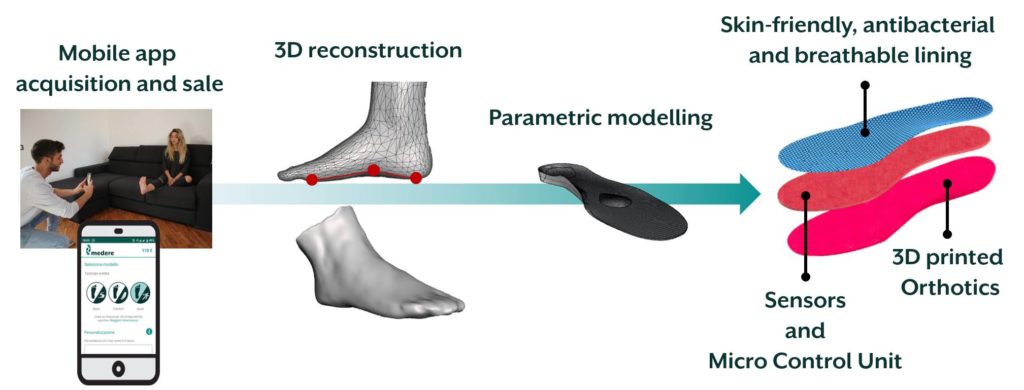
Medere, with the support of MADE Competence Center, aims at developing STEP (Sensorised insoles for therapeutic monitoring of patients) project to release a system for real-life patient monitoring based on custom-made sensorised insoles. The main objective of the project is to provide a system for diagnostics and therapeutic monitoring inside custom-made insoles realised with digital acquisition and 3D printing. In this way, data recorded in real-life will be analysed and elaborated with dedicated software and shared securely with clinicians, enabling the possibility to provide an indispensable service, and not present today, for doctors and patients.
Expected results
Medere and MADE aim at the development and validation of a fully operating system that will be ready for certification and EU commercialisation.